On Tuesday, US health officials at the FDA issued approval for a new drug treatment option for mild and early cases of dementia caused by Alzheimer’s.
With this approval, this drug from an American company becomes only the second drug approved by the FDA in decades that has proven to slow down the progression of the disease.
Recent History

Federal health officials have closely watched the development of the drug by Eli Lilly and Co. which was seeking approval from the Food and Drug Administration (FDA).
Last month, a panel of FDA advisors was convinced of evidence that showed the drug “Kisunla” had a modest effect of slowing the decline of Alzheimer’s patients by about four to seven months and voted unanimously in support of its benefits.
Drug Conference

In June, company representative Dr. John Sims told reporters at a conference that this latest drug breakthrough gives hope to those struggling with this incurable disease.
“Finally there’s some hope, right, that we can talk about,” said Sims. “We don’t cure the disease. Diabetes doesn’t have a cure either — it doesn’t mean you can’t have very meaningful treatments for patients.”
FDA Approval

This week, after the previous praise the drug received, the FDA finally approved Kinsunla for treating mild and early cases of Dementia.
While this drug can slow the progression of the disease, it does not come without a cost. Patients will need to submit to regular IV infusions and risk potentially dangerous side effects like brain swelling.
Different Options For Patients

While the upsides of the drug may seem minimal to some, physicians are lauding the drug approval which gives some hope for researchers after countless experimental treatments have failed to make progress for decades.
“I’m thrilled to have different options to help my patients,” said Dr. Suzanne Schindler, a neurologist at Washington University in St. Louis. “It’s been difficult as a dementia specialist — I diagnose my patients with Alzheimer’s and then every year I see them get worse and they progress until they die.”
Second Drug

With the latest approval by the FDA, Kisunla will become only the second approved drug for mitigating the effects of Alzheimer’s.
Last July, a similar drug by the Japanese drug company Esai became the first such drug to receive FDA approval.
What is Alzheimer’s?
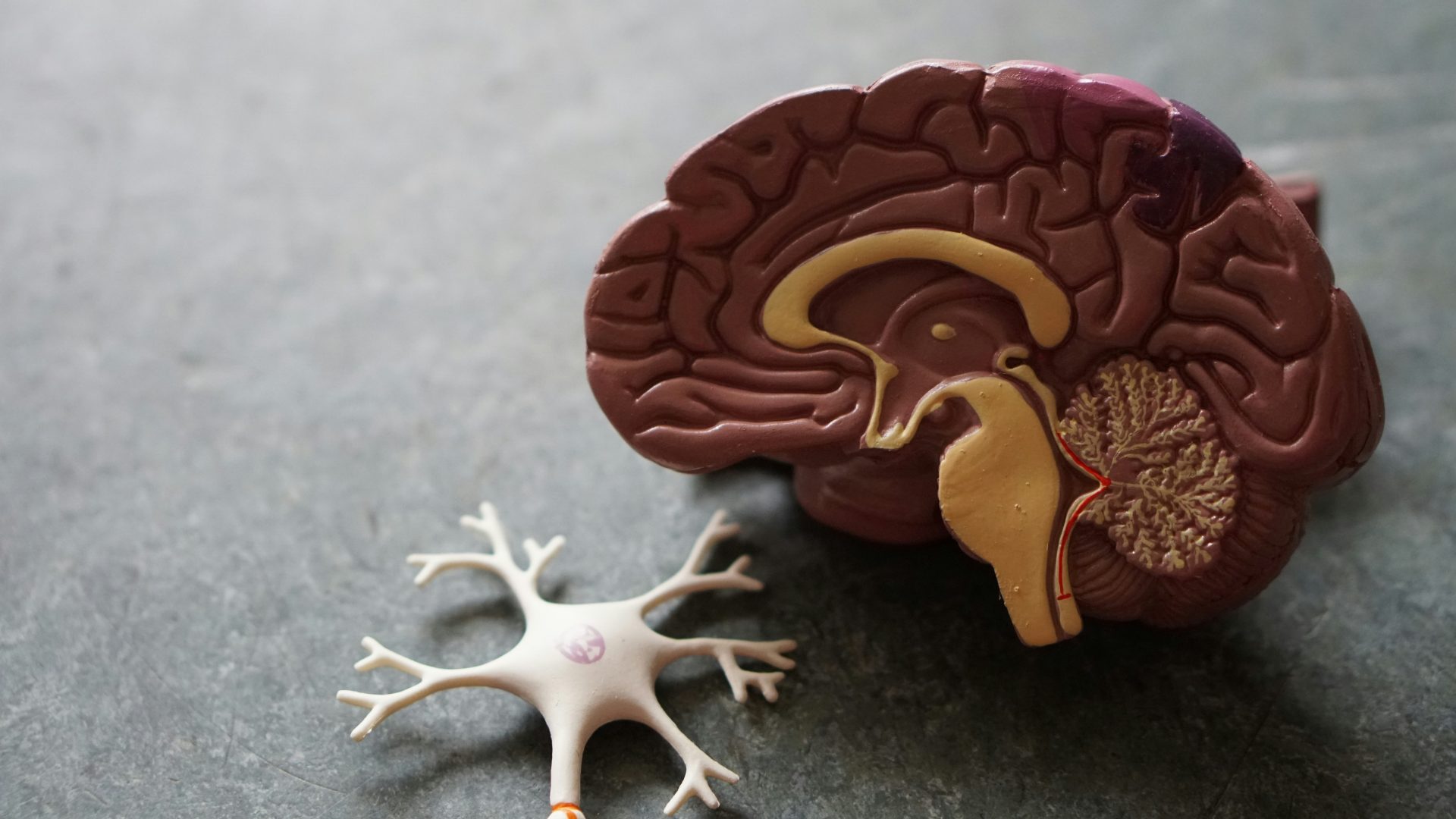
Alzheimer’s is a disorder that inflicts progressive damage to the brain, leading to dementia, memory loss, and a reduction in cognition.
As the disease progresses, parts of the brain shrink and get inflamed, causing permanent damage as neurons in the brain lose connections to each other, eventually leading to neuron death.
Why is Alzheimer’s an Incurable Disease?

No known cure has been found for the progression of Alzheimer’s disease, partly because scientists still don’t fully understand how it works.
Researchers have been able to correlate the effects of aging, genetic predisposition, and environmental factors with an increased liklihood of developing Alzheimer’s, but have not yet isolated the set of circumstances that causes it to start developing.
Hidden Disease

One difficult thing about treating Alzheimer’s while it is still in its early stages is that it can be years of disease progression before the first noticeable symptoms show up.
Current research into the disease is examining the role of two proteins in the brain which are thought to contribute to the loss of communication between brain cells.
How Do The Drugs Work?

Both Kisulna and the Japanese drug Leqembi work through the utilization of antibodies made in a laboratory that are injected through an IV into a patient. These antibodies were designed to target proteins which can cause a plaque buildup in an Alzheimer patient’s brain
The new benefit of Kisulna over the previous drug is that IV treatments only need to be done once a month instead of every two weeks.
Are These Drugs Covered Under Medicare?

After receiving FDA approval last year, the previous drug Leqembi became eligible for coverage under Medicare Part B and some private insurance plans.
However, Lequembi is a pricy drug, and Medicare Part B only pays for 20% of its cost. According to the National Council on Aging, the cost of Leqembi is $26,500 per year.
Possibility of Kisunla’s Coverage
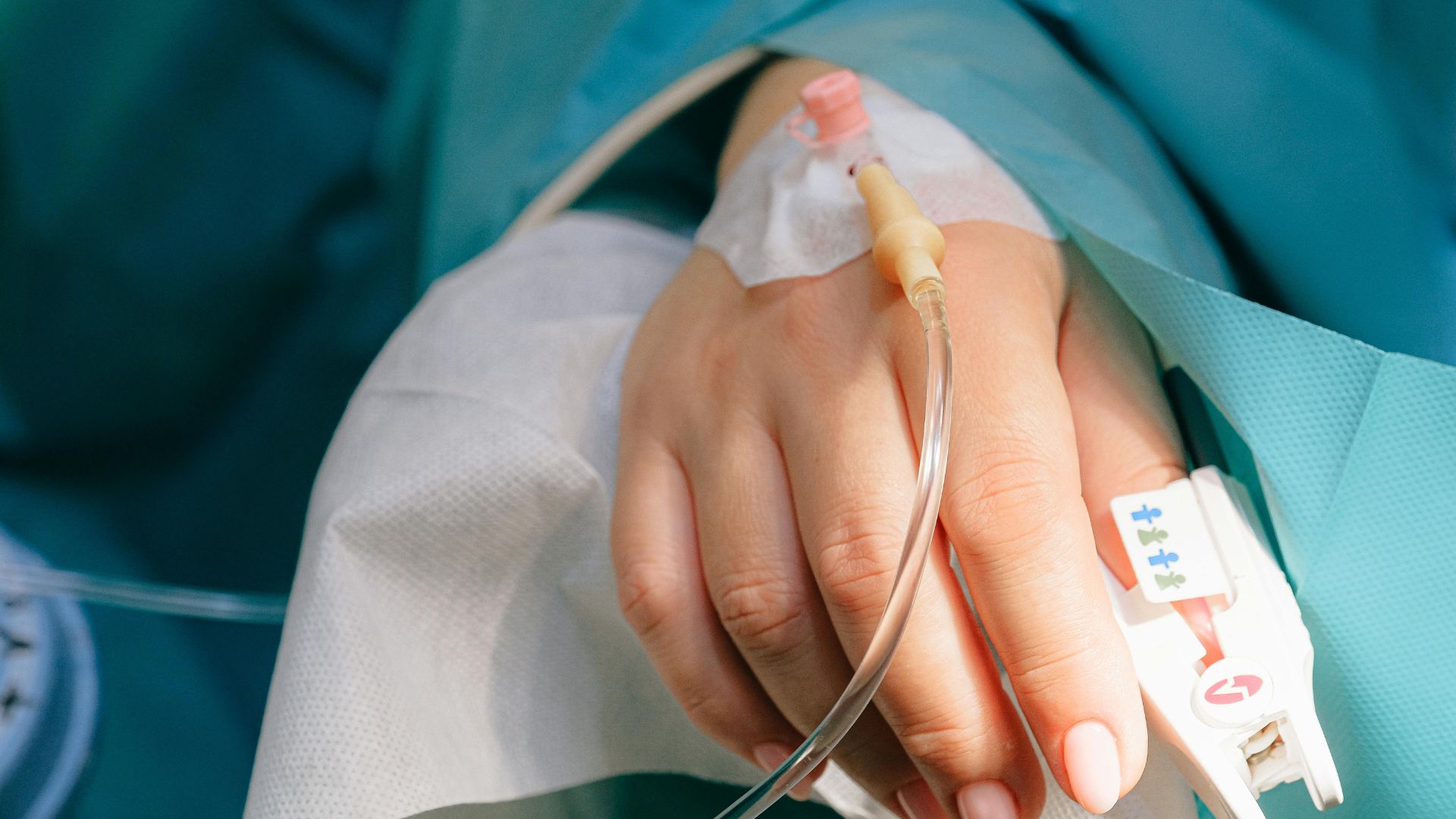
Similar to the drug last year, it is likely Kisunla will also see some form of cost reduction through Medicare health coverage which stats show would have a similar price tag.
Eli Lilly says that the drug will cost $12,522 for a six-month treatment and up to $48,696 for an 18-month treatment.
